Abstract
RUNX1 is a member of the core-binding factor family of transcription factors and is imperative for establishing definitive haematopoiesis. Mutated RUNX1 is an adverse risk factor for myelodysplastic syndrome (MDS) and acute myeloid leukaemia (AML). Meanwhile, high expression of RUNX1 is correlated with dismal prognosis in cytogenetically normal AML patients and critical for maintaining leukemic stem cells across AML genetic subgroups. However, the clinical relevancy of RUNX1 expression in MDS patients remains elusive. This study aimed to investigate the prognostic and biologic impacts of RUNX1 expression in MDS patients. We recruited 341 primary MDS patients who had enough bone marrow (BM) samples for RNA and next-generation sequencing. We first examined the difference in RUNX1 expression among patients with wild RUNX1 and N-terminal or C-terminal RUNX1 mutation. Among the 341 patients, 54 (15.8%) harboured RUNX1 mutation, 15 (27.8%) in N-terminal and 39 (72.2%), C-terminal. Patients with C-terminal RUNX1 mutant had higher RUNX1 expression than those with N-terminal RUNX1 mutant or wild RUNX1 (p<0.001 ). Patients were then divided into two groups with higher- and lower-RUNX1 expression (median as cut-off). Higher RUNX1 expression was closely associated with lower platelet counts, higher blast percentages in the BM and peripheral blood and complex karyotypes at diagnosis. Patients with higher-RUNX1 expression were more frequently categorized into higher-risk groups based on the revised international prognosis scoring system (IPSS-R). Higher RUNX1 expression was intimately associated with ASXL1, NPM1, RUNX1, SRSF2, STAG2, TET2, TP53, and ZRSR2 mutations, whereas lower RUNX1 was associated with SF3B1 mutation.
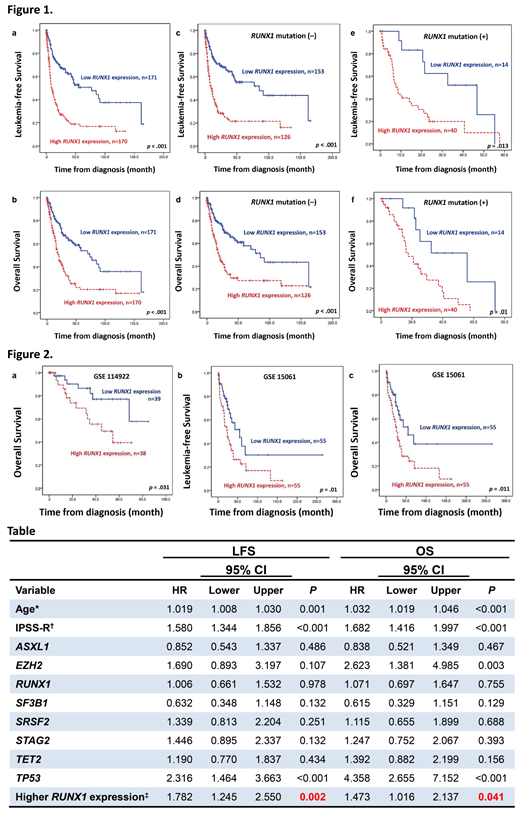
Regarding survival, we first examined the impact of RUNX1 mutation on survival in this cohort. As expected, patients with mutated RUNX1 had significantly inferior leukaemia-free survival (LFS) and overall survival (OS) than those with unmutated RUNX1 (p=0.007, and p=0.008, respectively). We then explored the effects of RUNX1 expression on patients' survival. Patients with higher RUNX1 expression had significantly inferior LFS and OS than those with lower expression (both p<0.001, Figure 1a and 1b). We further interrogated RUNX1 expression and mutation statuses for risk stratification. The higher-RUNX1 group consistently had shorter LFS and OS than the lower-RUNX1 group no matter RUNX1 was mutated or not (Figure 1c-1f). Subgroups analysis revealed the same findings in IPSS-R lower-risk (very low, low, and intermediate-risk) and IPSS-R higher-risk (high and very high risk) subgroups (all p<0.05). Moreover, time-dependent ROC curves indicated that RUNX1 expression had better predictive power for LFS and OS than RUNX1 mutation. In multivariate analysis, higher RUNX1 expression appeared as an independent adverse risk factor for LFS and OS irrespective of age, IPSS-R, and mutations in ASXL1, EZH2, RUNX1, SF3B1, SRSF2, STAG2, TET2, and TP53 (Table). The prognostic significance of RUNX1 expression was further validated in two external public cohorts, GSE 114922 and GSE15061. Patients with higher-RUNX1 expression consistently had significantly inferior survival than those with lower-RUNX1 expression (Figure 2). Bioinformatic analysis revealed that higher-RUNX1 patients had more robust IL-17 and MAPK signallings but exhausted antioxidant activities and antimicrobial humoral responses. In summary, we present the characteristics and prognosis of MDS patients with various RUNX1 expressions and propose that RUNX1 expression can complement RUNX1 mutation in MDS prognostication, wherein patients with wild RUNX1 but high expression may need more proactive treatment.
Tien: AbbVie: Honoraria; Celgene: Honoraria, Research Funding; Novartis: Honoraria. Chou: Abbvie: Honoraria, Other: Advisory Board, Research Funding; Celgene: Honoraria, Other: Advisory Board, Research Funding; IQVIA: Honoraria, Other: Advisory Board; Pfizer: Honoraria, Other: Advisory Board; Novartis: Honoraria, Other: Advisory Board; Bristol Myers Squibb: Honoraria, Research Funding; Kirin: Honoraria, Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal